Last Updated : April 29, 2025
Overview
In Canada, coverage for prescription drugs exists through an array of public and private drug plans.
The pan-Canadian Advisory Panel on a Framework for a Prescription Drug List (Advisory Panel) is creating a recommended framework for developing a potential pan-Canadian prescription drug list, or formulary.
The Advisory Panel’s non-binding recommendations are intended to add to the national conversation about ensuring Canadians have access to prescription drugs.
The mandate of the Advisory Panel is to:
- recommend principles and a framework for developing a pan-Canadian prescription drug list
- recommend an initial list of commonly prescribed drugs and a transparent way to add to that list
- consult with key stakeholders and health system partners, including federal, provincial, and territorial governments; patients; clinicians; industry; and others.
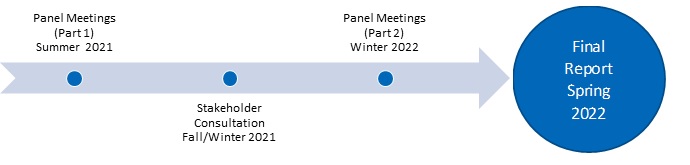
The Advisory Panel’s non-binding recommendations will be completed by the end of Spring 2022. The Advisory Panel’s final report will be submitted to Health Canada, shared with provincial and territorial governments, and made available publicly.
Project Timeline
Consultation Process
On behalf of the Advisory Panel, we invite interested parties to provide input on a proposed framework for a potential pan-Canadian formulary. The Advisory Panel prepared a discussion paper to contribute to the dialogue around this work. To provide input, please submit your responses and comments using the online form. The consultation period will close by end of business day on February 25, 2022.
Feedback Received
On behalf of the Advisory Panel, we would like to thank all respondents for your active participation and input in the consultation process. We received comments from many groups including patient groups, health care professionals, individuals from clinical societies, government and related agencies, associations, pharmaceutical companies, device companies, private insurance companies, researchers, consultants, and others.
As part of our commitment to our principle on transparency, all comments received by the consultation deadline are publicly posted.
Final Report: Building Toward a Potential Pan-Canadian Formulary
We’re pleased to announce the release of Building Toward a Potential Pan-Canadian Formulary: A Report From the Advisory Panel.
This report is intended to contribute to current conversations by decision-makers and others regarding a potential pan-Canadian formulary within a national pharmacare program, and the issues related to access, affordability, and appropriate use of prescription drugs in our health care systems.
Pan-Canadian Advisory Panel Members
The Advisory Panel is composed of 2 co-chairs and 12 members. Its members were recruited from across Canada and represent diversity across gender, culture, and race. The Advisory Panel brings together a range of expertise and experience, including health care providers (nursing, pharmacy, and medicine), persons with lived and living experience, and individuals with backgrounds in ethics and health policy.
Panel Members of the Pan-Canadian Advisory Panel on a Framework for a Prescription Drug List
| Name | Province | Bios |
|---|---|---|
| Co-Chairs | ||
|
Alexandra King
BSc, BBA, MD, FRCPC |
Saskatchewan |
Dr. Alexandra King is an internal medicine specialist with a focus on HIV/AIDS, hepatitis C, and HIV and oft-related conditions. Alexandra is a member of the Nipissing First Nation (Ontario) and the Cameco Chair in Indigenous Health and Wellness at the University of Saskatchewan. |
|
Allen Lefebvre
|
Saskatchewan |
Allen Lefebvre has served in several management and volunteer leadership capacities primarily in the charitable and not-for-profit sectors. Allen is a long-standing public member on our Canadian Drug Expert Committee and has been a public representative on the Drug Advisory Committee of Saskatchewan since 2010. |
| Members | ||
|
Stéphane Ahern
MA, MD, FRCPC, PhD |
Quebec |
Dr. Stéphane P. Ahern is an adult intensive care and general internal medicine specialist at CIUSSS de l’Est-de-l’Île-de-Montréal – Hôpital Maisonneuve-Rosemont and an Associate Professor in the Faculty of Medicine at the University of Montreal. He holds a master’s degree in philosophy, with a focus on clinical ethics, and a PhD in clinical science. For many years, he has been interested in the evaluation of drug reimbursements for the Institut national d’excellence en santé et en services sociaux Standing Scientific Committee on Entry on the List of Medications. |
|
Shawn Bugden BSc (Hons), BSc (Pharm), MSc, PharmD |
Newfoundland and Labrador |
Dr. Shawn Bugden is Dean at the School of Pharmacy at Memorial University of Newfoundland and Labrador and an Adjunct Professor at the College of Pharmacy at the University of Manitoba. Shawn is also on the Board of Directors for the Canadian Pharmacists Association. Shawn has expertise in hospital pharmacy, health policy, pharmacoepidemiology, pharmacovigilance, and pharmacoeconomic evaluation. |
|
Connie Côté
MA |
Ontario |
Connie Côté is the Chief Executive Officer of the Health Charities Coalition of Canada. Connie has extensive experience in the not-for-profit health sector. Connie’s area of interests include health policy, health research, and promoting the voices of patients and caregivers. Connie is a current care provider. |
|
Elsie Duff
BScN, RN, NP, MEd, PhD |
Manitoba |
Dr. Elsie Duff is a nurse practitioner and an assistant professor in the College of Nursing, Rady Faculty of Health Sciences, University of Manitoba. Elsie has lived and worked in rural and remote locations in Manitoba and has extensive expertise as a first-line provider of primary health care and virtual care, episodic care, disease prevention, and promotion of health. Elsie has served on several university and community boards and committees. |
|
Bashir Jiwani MA, PhD |
British Columbia |
Dr. Bashir Jiwani is Executive Director, Ethics and Diversity Services, at the Fraser Health Authority. Recent areas of focus include allocating financial resources to support COVID-19 response, producing decision-making structures and processes for expensive drugs for rare diseases, and creating a gender-inclusive climate. Bashir currently serves on the Drug Benefit Council for BC Ministry of Health; the Expensive Drugs for Rare Diseases Committee for BC Ministry of Health; the Core Team for the Aga Khan University Thinking Group on Ethics, Stem Cell Science, and Regenerative Medicine; and as a consultant for the Ismaili Tariqah and Religious Education Board of Canada. Bashir is President-Elect of the Canadian Bioethics Society. |
|
Diane McArthur
BPR, MBA |
Ontario |
Diane McArthur is a former Ontario public servant. Diane was Ontario’s first Chief Talent Officer and Associate Deputy Minister with the Centre for Leadership and Learning in the Treasury Board. Diane was also the Assistant Deputy Minister and Executive Officer, Ontario Public Drug Programs Division. Diane is currently an active member on several boards in the broader public sector sharing her experience in government relations, governance, and program administration. |
|
Andrew Pinto
MSc, MD, CCFP, FRCPC |
Ontario |
Dr. Andrew Pinto is the founder and director of the Upstream Lab, a research team focused on integrating health and social care, population health management, and data to enable proactive care. Andrew is a Public Health and Preventive Medicine specialist and family physician at St. Michael’s Hospital, and a scientist at MAP at the Li Ka Shing Knowledge Institute, Unity Health Toronto. Andrew is an Associate Professor at the University of Toronto, appointed to the Department of Family and Community Medicine, the Institute for Health Policy, Management and Evaluation, and the Dalla Lana School of Public Health. |
|
Sheri Roach
RN, MN, MHA |
Nova Scotia |
Sheri Roach is a registered nurse with a background in direct care, health care leadership, policy, and education. Sheri is a faculty member at Dalhousie University’s School of Nursing and sits on the Board of Directors for the Canadian Nurses Association.
|
|
Adil Virani
BSc (Pharm), PharmD |
British Columbia |
Dr.Adil Virani is a manager with Lower Mainland Pharmacy Services and has hospital, community, and university experience in British Columbia and Nova Scotia. Adil has been a member of our Canadian Drug Expert Committee and the Patented Medicine Prices Review Board’s Human Drug Advisory Panel since 2008.
|
|
Cornelia (Nel) Wieman
MSc, MD, FRCPC |
British Columbia |
Dr. Cornelia (Nel) Wieman is Canada’s first female Indigenous psychiatrist. Nel is President of the Indigenous Physicians Association of Canada and Acting Deputy Chief Medical Officer in the Office of the Chief Medical Officer at the First Nations Health Authority in British Columbia. |
|
Sam Wong
MD, FRCPC |
Alberta and the Northwest Territories |
Dr. Sam Wong is a pediatrician who works part-time in Yellowknife in general pediatrics and travel clinics for a variety of communities in the Northwest Territories and Nunavut. Sam is also a part-time hospital pediatrician at the Stollery Children’s Hospital in Edmonton. Most recently, Sam was the chair of the First Nations, Inuit, and Métis Health Committee of the Canadian Pediatric Society. Sam is currently president of the Canadian Pediatric Society (2020–2021).
|
|
Yan Yu
MD, MPP, MBA, CCFP |
Alberta |
Dr. Yan Yu is a family physician in Alberta and the Northwest Territories and is a member of the College of Family Physicians of Canada Board of Directors. Yan practices in numerous clinical settings, such as community-based family medicine clinics, long-term care homes, hospitals, and rural emergency rooms. Yan’s clinical interests include working in emergency psychiatry and providing care to rural, remote, and Indigenous communities.
|
FAQs - (Responses to FAQs are based on the Advisory Panel’s draft recommendations, as outlined in the Discussion Paper)
About the Advisory Panel
1. Who is on the Advisory Panel?
The names, biographies, and conflict of interest declarations of the 14 members of the Advisory Panel are available above.
The Advisory Panel comprises:
- 2 co-chairs (a physician and a caregiver member)
- 1 ethicist
- 1 former Assistant Deputy Minister–level drug plan lead
- 1 nurse practitioner and 1 registered nurse
- 1 representative of a national health charity
- 2 pharmacists
- 5 physicians (generalists and specialists).
2. How were the Advisory Panel members chosen?
We selected the Advisory Panel members with advice and guidance from its federal, provincial, and territorial funders.
The Advisory Panel comprises 2 co-chairs and 12 members. Its members were recruited from across Canada and represent diversity across gender, culture, race, and geographic area. The panel brings together members with a range of expertise and experience, including health care providers (nursing, pharmacy, and medicine), persons representing those with lived and living experience, persons working with Indigenous and other communities often made vulnerable through a combination of social and economic policy, and individuals with backgrounds in ethics, health policy, and drug plan leadership.
Scope of Work and Deliverable
3. What is a formulary?
A formulary typically contains a list of prescription drugs and other products that could be covered by a health plan. It generally contains a description of each product that is listed and may also contain information to support prescribing, dispensing, and administration of the product as well as any interchangeability between products. The general purpose of a formulary is to ensure that the treatments that are used are safe, effective, affordable, and cost-effective (i.e., takes into account how well a drug or technology works in relation to how much it costs).
4. What is the goal of a potential pan-Canadian formulary?
The goal of a potential pan-Canadian formulary is to include a broad range of safe, effective, evidence-based drugs and related products that meet the health care needs of Canada’s diverse population. A potential pan-Canadian formulary could help make prescription drugs more accessible to all people living in Canada, especially to those who currently do not have access for reasons beyond their control, including both historic and contemporary inequities.
5. What is meant by “related products” and why are these products being considered for listing in addition to pharmaceuticals in a pan-Canadian formulary?
The term related products refers to devices that assist with delivery or administration of drugs and/or are necessary for the optimal use of drugs (e.g., spacer devices for metered dose inhalers or blood glucose strips). The Advisory Panel felt strongly that the inclusion of related products on a potential pan-Canadian formulary should be explored because this could help improve patient access and could potentially improve adherence with drug treatment. In many cases, these related products are covered through different programs within the health system, which makes accessing coverage difficult for patients. As such, a potential pan-Canadian formulary could be an opportunity to streamline the process, provide simplified access, and ultimately help patients access these types of products.
6. What is the overall objective of the Advisory Panel and how will the work on the pan-Canadian formulary fit into the broader discussion about prescription drug coverage in Canada?
The objective of the Advisory Panel is to contribute to the current dialogue regarding the development of a potential pan-Canadian formulary, including addressing issues related to access to prescription drugs and select related products for all people in Canada regardless of age, disability, gender, geography, race, or socioeconomic status, among other characteristics. In developing a potential pan-Canadian formulary, the following elements should be addressed:
- terms of coverage (i.e., eligibility criteria or who may be covered)
- processes for creating a list of drugs and related products (i.e., what is covered and why)
- ways to manage the formulary (i.e., how to maintain the list so it is based on the best available evidence)
- how it could be financed (i.e., who or what group funds it)
- who makes the decisions (i.e., whether the listing decision made by a group, organization, or designated individual, such as the minister of health or an executive officer of a drug program).
The Advisory Panel’s recommendations are meant to support 2 of these 5 elements; specifically, to develop processes for creating a list of drugs and related products and to highlight best practices for managing a formulary.
7. What is the Advisory Panel’s scope of work?
The Advisory Panel’s recommendations are meant to support a small component of the process to develop a potential pan-Canadian formulary. The scope of work for the time-limited Advisory Panel includes:
- develop principles and a framework that could guide the development of a potential pan-Canadian formulary
- create a proposed sample list of commonly prescribed drugs and selected related products as a test case based on a subset of therapeutic areas that could be included on a potential pan-Canadian formulary
- establish criteria and a transparent process that could expand the proposed sample list to other therapeutic areas, and guide how new products could be added and how a proposed list could be maintained over time
- consult with key stakeholders, including federal, provincial, and territorial governments; patients; clinicians; industry; and other interested parties.
8. What is out of scope for the Advisory Panel?
The exercise of developing a potential pan-Canadian formulary is complex, and the mandate of the Advisory Panel was limited. The Advisory Panel’s work did not include:
- an assessment of current drug plan processes or expectations about whether or how coverage on existing drug plans might be impacted by a potential pan-Canadian formulary
- the identification of governance structures to implement a potential pan-Canadian formulary (i.e., which organization or entity should oversee implementation of a potential pan-Canadian formulary or make funding decisions)
- a consideration of financing issues (e.g., funding allocation; financial contributions; funding models; budget scope, size, and amount; or individual drug plan budgets or projected estimates for those budgets)
- the terms for coverage (e.g., patient contributions such as copayments or deductibles) and patient eligibility, including status
- a consideration of the interplay between public and private insurance plans (i.e., coverage as first and second payor)
- other ongoing pharmaceutical initiatives (e.g., Health Canada’s Drugs for Rare Diseases Strategy).
About the Principles and Framework
9. How were the principles developed?
As a starting point for meeting preparation and orientation, the Advisory Panel was provided with a set of principles and definitions from the published literature. These were varied in theme and touched on areas related to disease prevalence and evidence of efficacy, safety, comparative cost-effectiveness, and health system feasibility. The principles were sourced from key Canadian documents such as the Canada Health Act as well as a limited literature search. This information was supplemented by a focused internet search for relevant grey literature and publications on principles regarding prescription drug access in the Canadian context.
10. What are the principles?
As part of developing a framework, the Advisory Panel recommended 6 guiding principles and accompanying definitions that would shape the overall system for a potential pan-Canadian formulary. The proposed principles are not ranked in order of importance nor are they independent from one another. Each influences, balances, supports, and, in some cases, builds on the others.
The 6 principles and accompanying definitions proposed by the panel are outlined in Table 1.
Table 1: Summary of Proposed Principles and Definitions
| Principle (important commitments the system must live up to) | Definition (in the context of a potential pan-Canadian formulary) |
|---|---|
| Universal and integrated | All people in Canada should have access to the prescription drugs they need regardless of their diversity characteristics (which include, but are not limited to, socioeconomic status, age, sex, gender, genetic characteristics, disability, geography, and membership in a cultural group). |
| Equitable | Equity recognizes that individuals have different circumstances that require variable allocation of resources to provide opportunities to achieve equal outcomes. Policies and processes for a potential pan-Canadian formulary should close gaps in access to prescription drugs, especially when the gaps arise from unintended consequences of policies that may create variation in access. |
| Effective and high quality | A potential pan-Canadian formulary should strive to provide access to Canadians to meet the highest standard of health and patient experiences. Choices should be based on an evaluation of the options and viewed in the context of benefit to patients and to the Canadian population as a whole. A potential pan-Canadian formulary should be monitored so that it can be continuously improved. |
| Sustainable | The people of Canada should benefit from a formulary management system that that maintains its own viability and supports long-term development and vision. |
| Efficient and timely | The process should minimize duplication of steps and ensure access to prescription drugs on the potential pan-Canadian formulary is provided in a seamless manner to ensure the right drug gets to the right patient at the right time. |
| Inclusive, transparent, and fair process | A potential pan-Canadian formulary should be developed and managed in collaboration with stakeholders, such as patients, people with lived and living experience including caregivers, health care providers, health organizations, governments, and industry. |
11. What are the considerations for inclusion, diversity, and equity?
Inclusion, diversity, and equity were the foundational principles in the recruitment of the Advisory Panel, as well as the development and application of the framework. Key aspects outlined by the Advisory Panel included:
- an acknowledgement that the framework and process must allow for a strong focus on universal access — access for all people in Canada across geographic and cultural contexts
- recognition that applying a population health perspective might put already disadvantaged populations further behind and not allow the needs of individual patients or communities to be adequately identified or addressed
- equity should be a key guiding principle, with an intent to acknowledge and help address the current inconsistent and systemically inequitable access to, and coverage for, drugs and related products that certain groups may disproportionately face barriers to access
- a potential pan-Canadian formulary could help make prescription drugs more accessible to all people living in Canada, especially to those who currently do not have access for reasons beyond their control, including both historic and contemporary inequities
- this work should not widen the gap between communities and groups
- the importance of incorporating evidence that considers diverse populations, perspectives, and experiences, and assessing value in a way that captures the experiences of a wide range of populations.
Approach to Developing a Potential Pan-Canadian Formulary
12. What approach did the Advisory Panel take to create a potential pan-Canadian formulary?
To create a potential pan-Canadian formulary, the Advisory Panel undertook a 3-stage approach in its deliberations as follows:
- Stage 1: Select a small sample list of commonly prescribed drugs and related products as a proof of concept for the process. Ensure that the guiding principles are followed while creating the proposed list.
- Stage 2: Review and revise the proposed list as appropriate, then apply the proposed criteria to other therapeutic areas in a subsequent future step to scale the process and expand the proposed list.
- Stage 3: Recommend criteria and processes for adding new drugs and related products once all therapeutic areas have been considered. Also suggest strategies to maintain a proposed list over time and to explore how this process could be integrated within the current system.
Stakeholder Engagement
13. How will interested parties be engaged?
On behalf of the Advisory Panel, we will reach out to stakeholders to solicit input and feedback.
The Advisory Panel is also hosting a 2-part webinar series.
The first session on January 18, 2022, will be to discuss recommendations for the development of a framework for a potential pan-Canadian formulary. You can register for the January webinar on the our website. The webinar will be recorded and made publicly available for those unable to attend the live session. A discussion paper summarizing the Advisory Panel’s interim recommendations and proposed process has been prepared and is available to download. During an online consultation period between January 11 and February 25, 2022, stakeholders will have an opportunity to submit their feedback to us through an online questionnaire.
The second stakeholder session will be organized in spring 2022 to share the comments that will help refine the report and the key changes that will be incorporated.
The webinars and opportunities to provide written submissions will be promoted through our regular communications channels.
To receive updates on engagement opportunities, please subscribe to our Weekly e-Alerts.
14. What will happen with the Advisory Panel’s recommendations?
The Advisory Panel’s recommendations will be completed by the end of spring 2022. After this, a final report will be submitted to Health Canada, shared with provincial and territorial governments, and made publicly available. The Advisory Panel’s recommendations will be non-binding. Governments will have the option to use the Advisory Panel’s recommendations to inform their discussions about a potential pan-Canadian formulary.
Last Updated : April 29, 2025














